
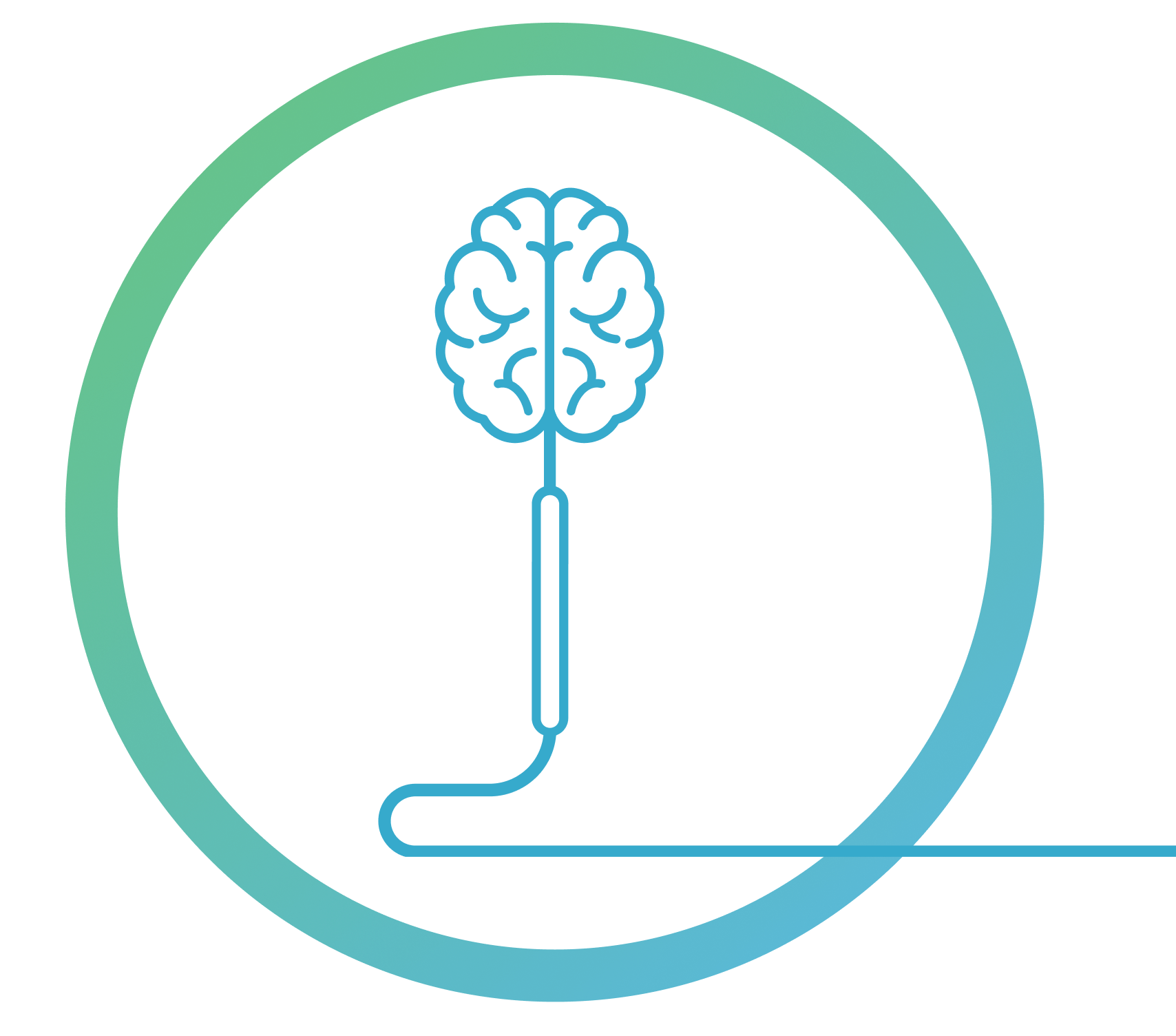
THE eShunt® SYSTEM
The eShunt® System is a new, investigational device for the treatment of NPH. The procedure to implant the shuntA shunt is a hollow tube that is placed in the brain during surgery to help drain excess cerebrospinal fluid. Shunts are commonly used to treat normal pressure hydrocephalus. is minimally invasive, meaning it can be done with only a small incision in the leg.
Currently, NPH is treated with traditional shunts, including the ventriculoperitoneal (VP) shunt. Traditional shunts come with certain challenges, including increased risk of infection, shunt failure, and other complications that can lead to multiple follow-up visits and more surgeries.
The US Food and Drug Administration has granted the eShunt® System a Breakthrough Device Designation. This program recognizes certain medical devices that may provide more effective treatments for serious conditions.
